Abstract
Background: Patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) after prior autologous stem cell transplant (ASCT) or chimeric antigen receptor T-cell (CAR-T) therapy have poor outcomes with limited treatment options. Bruton's tyrosine kinase (BTK) inhibitors are safe and effective agents in subsets of DLBCL with chronic active B-cell receptor (BCR) signaling, but durations of remission are short. Acalabrutinib, a highly selective, covalent, potent next-generation inhibitor of BTK (Calquence ® prescribing information [USPI]), is approved for the treatment of mantle cell lymphoma and chronic lymphocytic leukemia and is being explored in combination with other rational targeted agents in DLBCL. High levels of signal transducer and activator of transcription 3 (STAT3) expression and activation have been preferentially detected in activated B-cell DLBCL (Ding et al, 2008), and inhibition of STAT3 has suppressed DLBCL in preclinical models (Scuto et al, 2011). A phase 1b study demonstrated the safety and tolerability of AZD9150 in DLBCL with some evidence of clinical activity including 2 complete responses (CRs) and 2 partial responses (PRs) in 27 patients (Reilley et al, 2018). We report results from one of the arms of a phase 1 master protocol PRISM study (NCT03527147: A Platform Protocol for the Treatment of Relapsed/Refractory Aggressive Non-Hodgkin's Lymphoma), which evaluated combination therapy of acalabrutinib with the anti-STAT3 allele-specific oligonucleotide AZD9150 in patients with R/R DLBCL.
Methods: Study participants were patients with R/R DLBCL aged ≥18 years with Eastern Cooperative Oncology Group performance status ≤2 and after ≥1 line of prior chemo-immunotherapy (including patients failing or ineligible for ASCT or CAR-T therapy). Starting on cycle 1 day 1 (C1D1), acalabrutinib was administered at 100 mg twice daily until disease progression or discontinuation. AZD9150 200 mg was administered as a 1-hour intravenous (IV) infusion on D1, D3, and D5 of C1, followed by weekly infusions (starting D8C1 and beyond). The primary endpoint was safety, and there was a dose-limiting toxicity (DLT) analysis after 6 subjects completed D28. Disease response (secondary endpoint) was assessed using Response Evaluation Criteria in Lymphoma 2017. Exploratory analyses included longitudinal peripheral blood and tumor tissue samples including immunophenotyping by flow cytometry and gene expression from total blood RNA (using the Nanostring - PanCancer IO 360™ Panel). Molecular classification at baseline was explored using available tumor tissue samples by LymphGen classification (Wright et al, 2020). Circulating tumor DNA (ctDNA) analysis was conducted using whole gene sequencing and a custom next-generation sequencing panel (AZHeme 600; Collins GP et al, 2021) and correlated with clinical response.
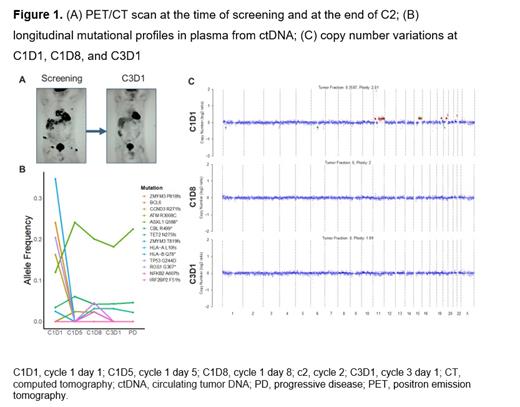
Results: A total of 17 patients were enrolled; median age was 72 (range, 34-88) years and 47% (8/17) of patients were ≥75 years. Median number of prior lines of therapy was 2 (range, 1-6). Overall response rate was 24% with a CR rate of 12%. The most commonly reported adverse events (≥25%) of any grade independent of study drug attribution were anemia, AST/ALT elevation, thrombocytopenia, neutropenia, and fatigue. One DLT of grade 3 AST/ALT increase was observed, and there were no treatment-related deaths. Total cell-free DNA (cfDNA) levels and the allele frequency of mutations within ctDNA correlated with treatment response. One patient achieved an early CR to therapy after C2 (Figure 1A), which correlated with dynamic changes in ctDNA (Figure 1B) including the disappearance of copy number changes (Figure 1C). Patients with persistent copy number changes detected (≥1 alteration) as early as C1D8 were less likely to respond to acalabrutinib in combination with AZD9150. Cell of origin phenotype of DLBCL or genetic subtype did not correlate with response. The pharmacokinetics of acalabrutinib, ACP-5862 (active metabolite of acalabrutinib), and AZD9150 were generally consistent with historical monotherapy profiles.
Conclusions: Targeting BTK and STAT3 is safe and tolerable in relapsed DLBCL but has limited efficacy. Early clearance of copy number variations and decreased cfDNA levels were associated with clinical responses and may be a useful biomarker with targeted agents.
Munugalavadla: AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Nuttall: AstraZeneca: Current Employment, Current holder of individual stocks in a privately-held company. Burke: AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Acar: AstraZeneca: Current Employment; Bristol Myers Squibb: Current equity holder in publicly-traded company, Ended employment in the past 24 months. White: AstraZeneca: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Udriste: AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Sharma: AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Dougherty: Pfizer: Current equity holder in publicly-traded company; AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Research Funding. Flinn: Incyte: Research Funding; MorphoSys: Consultancy, Research Funding; AbbVie: Consultancy; Great Point Partners: Consultancy; Iksuda Therapeutics: Consultancy; IGM Biosciences: Research Funding; Karyopharm Therapeutics: Research Funding; Juno Therapeutics: Consultancy, Research Funding; Janssen: Research Funding; Merck: Research Funding; Loxo: Research Funding; KITE Pharma: Consultancy, Research Funding; Acerta: Research Funding; Gilead Sciences: Consultancy, Research Funding; Hutchison MediPharma: Consultancy; Nurix Therapeutics: Consultancy; Agios: Research Funding; Calithera: Research Funding; Infinity Pharmaceuticals: Research Funding; Rhizen Pharmaceuticals: Research Funding; Century Therapeutics: Consultancy; Beigene: Consultancy, Research Funding; ArQule: Research Funding; AstraZeneca: Consultancy, Research Funding; Celgene: Research Funding; Constellation Pharmaceuticals: Research Funding; Curis: Research Funding; Forma Therapeutics: Research Funding; Forty Seven: Research Funding; Genentech: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; Portola Pharmaceuticals: Research Funding; Pharmacyclics: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Servier Pharmaceuticals: Consultancy; Takeda: Consultancy, Research Funding; Teva: Research Funding; TG Therapeutics: Consultancy, Research Funding; Trillium Therapeutics: Research Funding; Triphase Research & Development Corp.: Research Funding; Unum Therapeutics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Vincerx Pharma: Consultancy; Yingli Pharmaceuticals: Consultancy. Saba: AbbVie (venetoclax): Consultancy, Honoraria, Speakers Bureau; AbbVie, PCYC, Janssen (ibrutinib): Consultancy, Honoraria, Speakers Bureau; Kyowa Kirin (mogamulizumab-kpkc): Honoraria, Other: advisory board ; Kite: Honoraria, Other: advisory board ; TG Therapeutics: Honoraria, Other: advisory board ; Epizyme: Honoraria, Other: advisory board ; Karyopharm: Honoraria, Other: advisory board . Reagan: Genentech: Research Funding; Kite, a Gilead Company: Consultancy; Curis: Consultancy; Seagen: Research Funding. Collins: AstraZeneca: Honoraria, Research Funding; Amgen: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celleron: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck Sharp & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Pfizer: Honoraria; Novartis: Honoraria, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Speakers Bureau. Mortlock: ARTICA Therapeutics B.V.: Membership on an entity's Board of Directors or advisory committees; Anavo Therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Patel: Portola Pharmaceuticals: Research Funding; Vigeo: Research Funding; Vedanta: Research Funding; Verastem: Research Funding; Alexion, AstraZeneca Rare Disease: Other: Study investigator; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Exelixis: Membership on an entity's Board of Directors or advisory committees; Xencor: Research Funding; TopAlliance: Research Funding; Tesaro: Research Funding; Taiho: Research Funding; Stemline Therapeutics: Research Funding; Synthorx: Research Funding; Syndax: Research Funding; Ribon Therapeutics: Research Funding; Revolution Medicines: Research Funding; Qilu Puget Sound Biotherapeutics: Research Funding; Prelude Therapeutics: Research Funding; Placon Therapeutics: Research Funding; Phoenix Molecular Designs: Research Funding; Genentech/Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Research Funding; GlaxoSmithKline: Research Funding; H3 Biomedicine: Research Funding; Hengrui: Research Funding; Hutchinson MediPharma: Research Funding; Ignyta: Research Funding; Incyte: Research Funding; Jacobio: Research Funding; Jounce Therapeutics: Research Funding; Klus Pharma: Research Funding; Kymab: Research Funding; Loxo Oncology: Research Funding; Lycera: Research Funding; Mabspace: Research Funding; Macrogenics: Research Funding; Millennium Pharmaceuticals: Research Funding; Mirati Therapeutics: Research Funding; ModernaTX: Research Funding; Forma Therapeutics: Research Funding; Evelo Biosciences: Research Funding; Eli Lilly: Research Funding; EMD Serono: Membership on an entity's Board of Directors or advisory committees, Research Funding; Effector Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Cyteir Therapeutics: Research Funding; Curis: Research Funding; Ciclomed: Research Funding; Clovis: Research Funding; Checkpoint Therapeutics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Calithera: Research Funding; Boehringer Ingelheim: Research Funding; BioNTech: Research Funding; Takeda: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; ORIC Pharmaceuticals: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; LSK Biopartners: Research Funding; Seven and Eight Biopharmaceuticals: Research Funding; Merck: Research Funding; Bicycle Therapeutics: Research Funding; AstraZeneca: Research Funding; Artios Pharma: Research Funding; Aileron Therapeutics: Research Funding; Agenus: Research Funding; ADC Therapeutics: Research Funding; Acerta Pharma: Research Funding; Florida Cancer Specialists: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal